 |
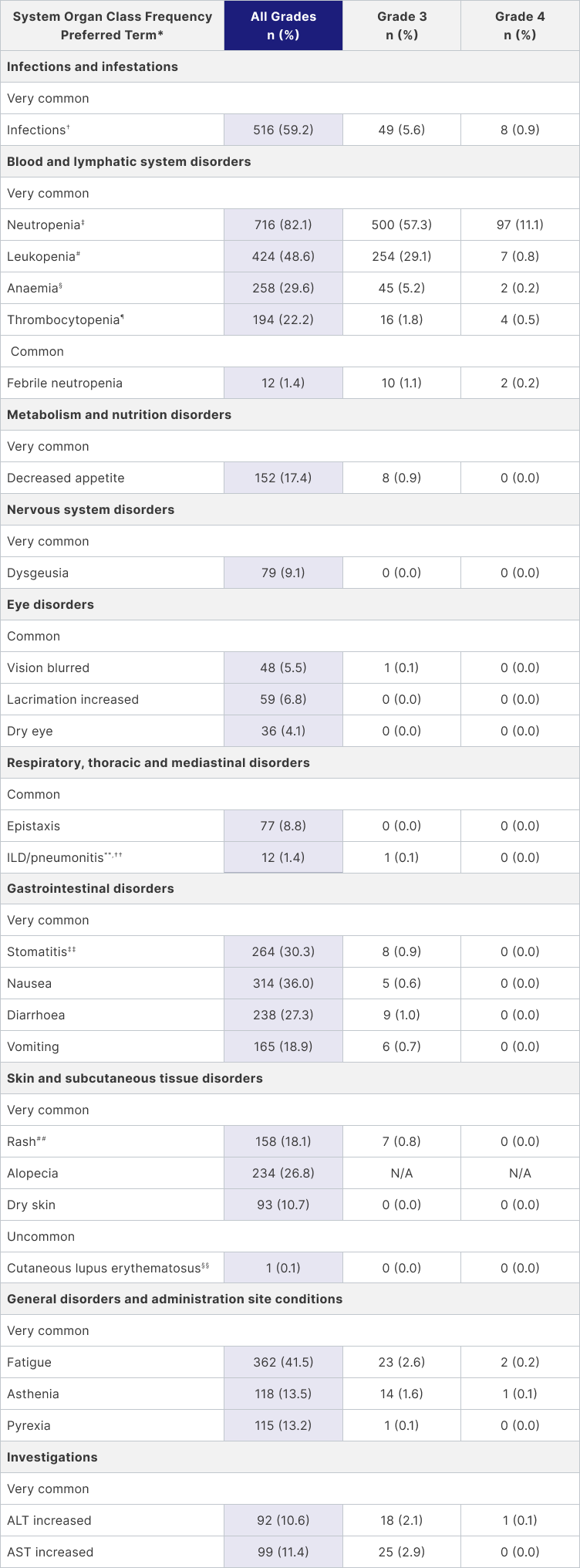
Adapted from Palbociclib Pfizer Summary of Product Characteristics.1
Effect on efficacy
Menu
Close
To report an adverse event, please contact [email protected]
 |
Effect on efficacy
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
This site is intended only for Rwanda health care professionals. The products discussed in this site may have different product labeling in different countries. The information provided is for educational purposes only.
© 2022 Pfizer Inc. All rights reserved.
Disclaimer: The product is not yet licensed by the Board of Health of Rwanda (Rwanda FDA). The product has however obtained prior Board of Health approval for supply to Accord program channels in Rwanda.