In an analysis of PALOMA-2 and -3, Palbociclib Pfizer in combination with letrozole and Palbociclib Pfizer in combination with fulvestrant in 1st line or later demonstrated a consistent safety profile in patients with visceral disease1,2
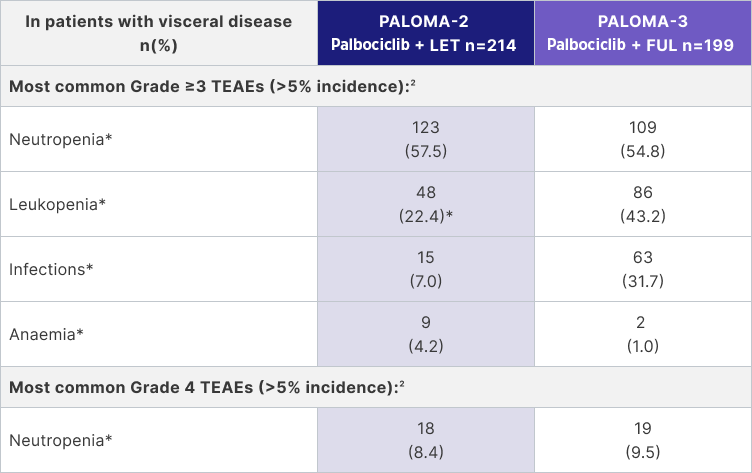 |
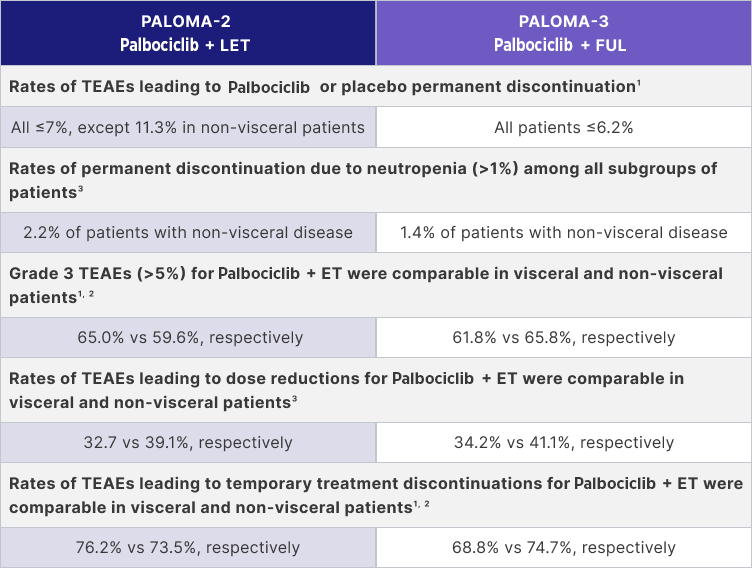 |