PALOMA-3 included a broad patient population, including 21% who were pre-/peri-menopausal and 21% of patients who had not received prior treatment for their metastatic disease (1st line)1
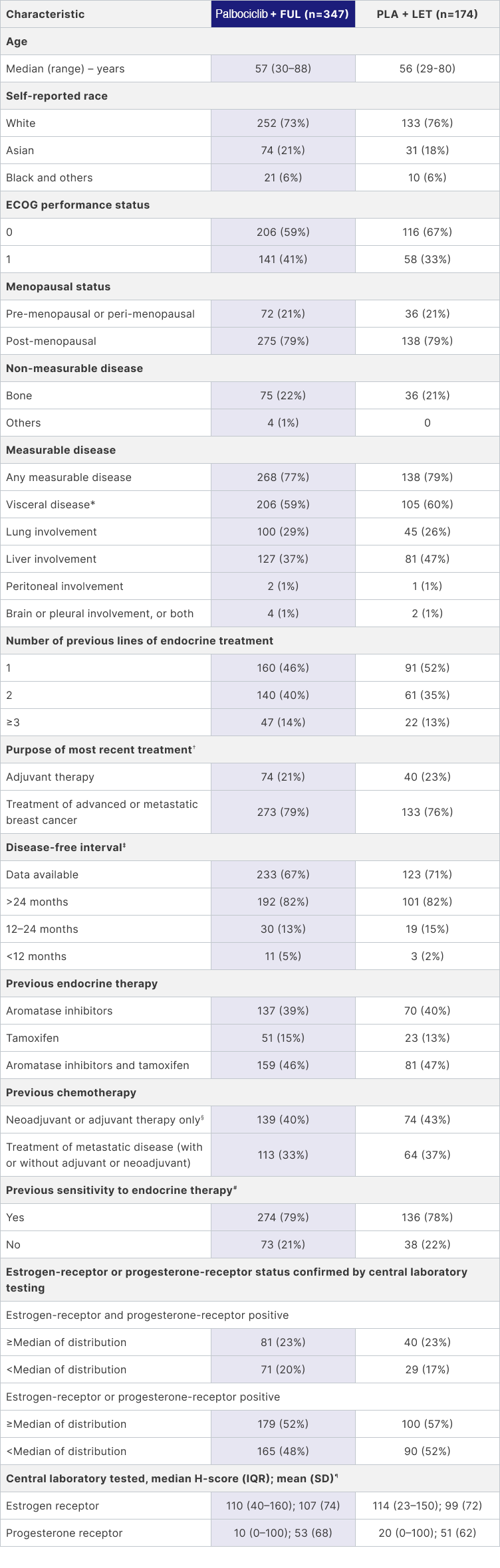 |
Data cut-off date: March 16, 2015. Data are number (%), unless otherwise specified. Because of rounding, some percentages do not total 100% when summed.
See PALOMA-2 patient baseline characteristics
HR+/HER2- = hormone receptor-positive, human epidermal growth factor receptor 2-negative; IQR = interquartile range;
ITT = intention to treat; mBC = metastatic breast cancer; n = number of patients; PLA = placebo; SD = standard deviation.
A broad range of patients with HR+/HER2- mBC are eligible for Palbociclib Pfizer + letrozole combination therapy